Knowledge for SPE Method Development
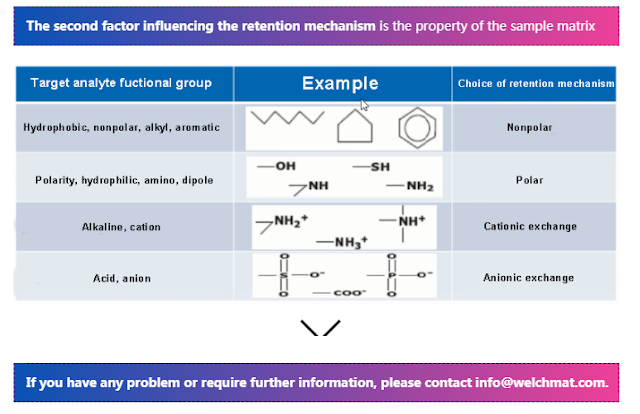
Reversed-phase extraction separation mainly uses the non-polar force between C-H bonds on SPE functional groups and C-H bonds of target compounds. In general, non-polar reversed-phase SPE columns are more suitable for extraction and separation of non-polar target compounds or target compounds with moderate polarity from polar matrices.
Target compounds adsorbed on nonpolar SPE columns by nonpolar forces can be eluted with nonpolar solvents, such as chloroform, cyclohexane, and ethyl acetate. The target compound can be eluted from the SPE column successfully as long as the solvent elution strength is sufficient to destroy the van der Waals force between the target compound and the non-polar functional group of the adsorbent. Even methanol with strong polarity has sufficient nonpolar force to elute many compounds. Sometimes single solvent fails to elute a hydrophobic target compound completely, dichloromethane : ethyl acetate (1:1 by volume) can be considered to use.
Under the reversed-phase solid phase extraction (SPE) mode, the polarity of the solvent system should decrease according to the order of sample solvent, rinsing solvent and elution solvent, while their elution strength increases gradually. It is necessary to ensure that the selected sample solvent cannot elute the target compound and the eluent selected should elute the interferences to the maximum extent without eluting the target compound. Eluent selected should also elute the target compound completely.
Polarity forces occur between the polar surfaces of SPE materials and the polar functional groups of the target compounds in the sample. Common adsorbents with polar forces are generally called normal phase chromatographic adsorbents. The polar force is stronger than the non-polar force, but less strong than the ionic force. Common polar functional groups include hydroxyl, amine, sulfhydryl and so on.
Nonpolar matrix environment is conducive to increasing polar forces between the adsorbent and the target compound, because nonpolar solvents do not have functional groups capable of forming hydrogen bonds with polar stationary materials. Therefore, in SPE with polar forces, the matrices of the sample are mostly non-polar, such as n-hexane, dichloromethane, rapeseed oil, etc., while the target compounds often contains functional groups with strong polarity.
Common polar stationary phase extraction materials include silica gel, alumina, Flori silica soil and bonded silica gel containing cyanide (CN), amino (NH2), diol (2OH).
Under the normal phase of SPE mode, the polarity of the solvent system should increase gradually according to the order of sample solvent, flushing solvent and elution solvent, and their elution strength should also increase gradually. It is necessary to ensure that the selected sample solvent cannot elute the target compound. The eluent selected should elute the interferences to the maximum extent without eluting the target compound. Eluent selected should also elute the target compound completely.
In addition to the ion-exchange solid phase extraction (SPE) technology introduced earlier, now there are so many adsorption mechanisms, such as reversed-phase and normal phase solid phase extraction (SPE). Which one should we choose in the practical application? The following content will have a specific analysis.
Since many compounds have multiple functional groups at the same time, it is necessary to consider which extraction mechanism is more favorable according to the properties of target compounds and interferences when selecting SPE mechanism. For example, 2-naphthylamine is a weakly basic compound (pKa=4.16), and can be cationic under certain pH condition. Meanwhile, the compound has hydrophobic non-polar functional groups and hydrophilic polar functional groups. In this case, the extraction mechanism that is conducive to the separation of the target compound from the disruptor should be selected according to the specific conditions of the sample matrix. If the sample matrix contains a large number of non-polar interfering impurities, the non-polar extraction mechanism should be avoided, and the pH of the sample should be adjusted to two pH units lower than its pKa, namely, pH=2.16. The cation exchange mechanism is recommended. On the contrary, if the sample contains a large number of cationic interfering impurities, the pH of the sample should be adjusted to 6.16 (two units higher than pKa), and non-polar extraction mechanism is more favorable.








Comments
Post a Comment